Manoop Chenchiliyan and his colleagues at Bar-Ilan University in Israel use calcium imaging to study the intracellular concentration of free ionized calcium (Ca²⁺). Ca²⁺ plays a key role in triggering a range of cellular events such as the contractile activity of muscle cells, neurotransmitter release, and oocyte fertilization.
“Understanding the spatial-temporal characteristics of Ca2+ signals are critically important in gaining the knowledge of the physiological regulations of the biological systems, which would provide new leads for pharmacological approaches,” said Chenchiliyan.
In a recent paper, the researchers demonstrated a new excitation light source that uses Prizmatix LEDs featuring high stability and fast switching to precisely match the excitation spectra of the commonly used Fura-2 Ca²⁺ indicator.
Monitoring Cellular Ca²⁺ Dynamics
Studying calcium as an intracellular messenger requires quantitative measurements of cytosolic free Ca2+ concentrations. Although no single approach exists for monitoring Ca²⁺ changes in all situations, imaging calcium-sensitive fluorescent indicators is the most widely used technique.
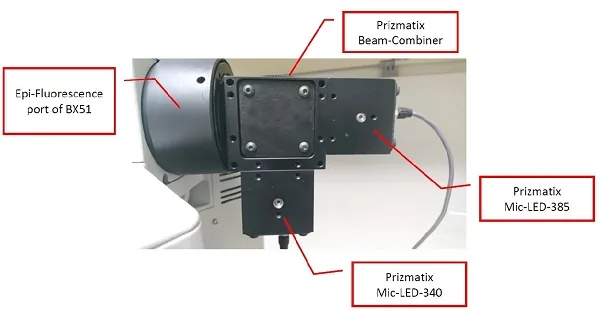
A new calcium imaging excitation source uses LEDs with high stability and fast switching to precisely match the excitation spectra of Fura-2.
One type of calcium indicator, known as ratiometric, shifts excitation or emission wavelengths when calcium is present, allowing the concentration of intracellular calcium to be determined from the ratio of fluorescence emission or excitation at distinct wavelengths.
Fura-2, one of the most common ratiometric fluorescent Ca2+ indicators, has an emission maximum at 510 nm and an excitation maximum that changes from 380 to 340 nm in response to calcium binding. Historically, a combination of arc lamp and monochromators have been used as the excitation light source for calcium indicator, but recently different combinations of LEDs such as 350 and 380 nm or 360 and 380 nm have also been used to excite Fura-2.
Using optimal excitation wavelengths
To precisely match Fura-2 excitation, the researchers used a new 340/385-nm excitation light source based on Mic-LED-340A and Mic-LED-385B LEDs from Prizmatix Ltd., Israel. The LEDs feature high intensity and stability as well as fast switching between wavelengths. One LED source had peak wavelength of 343.63 nm with a full width at half maximum of 12.41 nm and the other had a peak wavelength at 383.80 nm and full width at half maximum of 13.60 nm.
Prior to performing an actual experiment, the researchers used the Prizmatix Spectra-Viewer online software to simulate the excitation and emission spectra of Fura-2 with Ca²⁺ in free or bound state by inputting the optics used in the actual experiments. The software can be used to simulate any dualLED/ratiometric system.
Dynamic imaging of skeletal muscle cells.
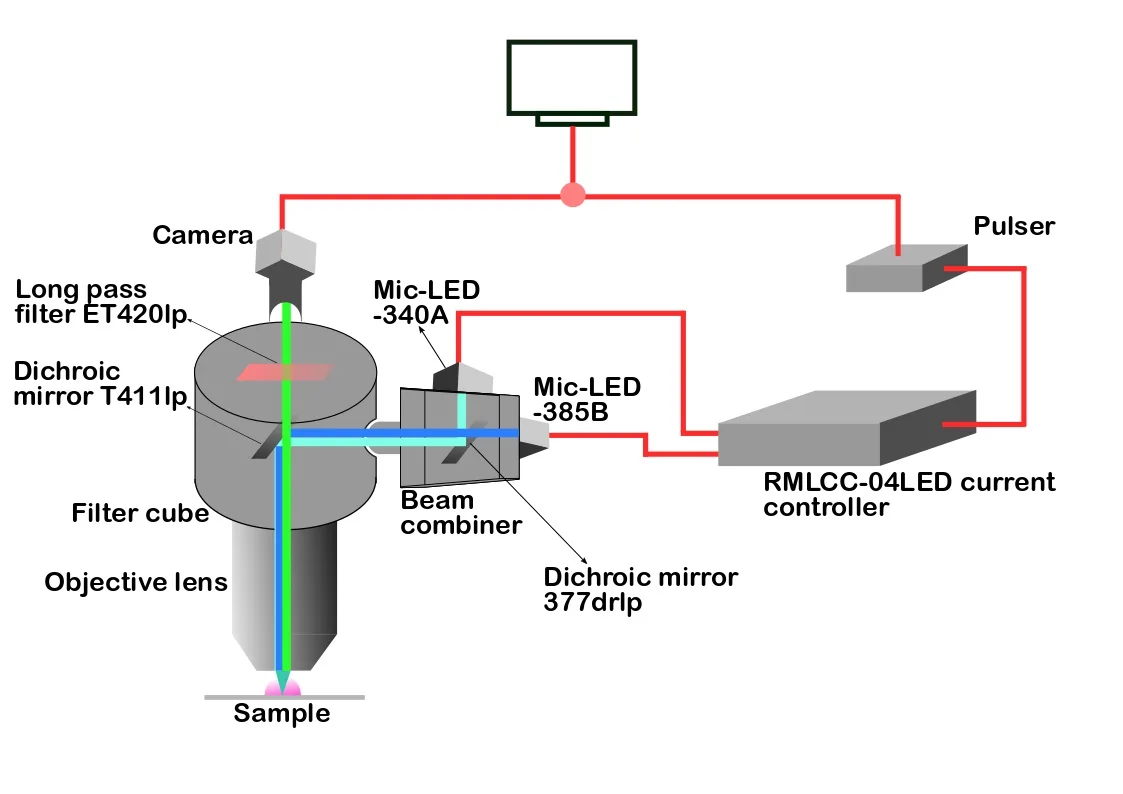
A programmable TTL pulse train generator synchronously triggered the camera and the LED pulses, helping the researcher to track transient intracellular free Ca2+ concentration changes in rat myotubes with high temporal resolution. Courtesy of Manoop Chenchiliyan, Bar-Ilan University.
The combined 340/385nm excitation light was coupled to the epifluorescence port of a wide field epifluorescence microscope and used to consecutively excite samples at 340 and 385nm. The researchers used the new LED light source to follow transient intracellular free Ca²⁺ concentration changes in rat skeletal muscle cells known as myotubes.
To take full advantage of the fast LED switching, the researchers used the Prizmatix programmable TTL pulse train generator to synchronously trigger the camera and the LED pulses, which was critical for obtaining information about the transient intracellular free Ca²⁺ concentration changes in rat myotubes.
After calibration, they were able to linearly measure intracellular Ca2+ levels between approximately 33 nM to 665 μM with high temporal resolution using the new LEDs as the excitation light source. This range of linearity in Ca²⁺ concentration was sufficient to precisely measure the intracellular free Ca²⁺ concentration changes in rat myotubes.
“The light source optimally matches the excitation wavelengths of either calcium-free or bound states of Fura-2,” said Chenchiliyan. “Our work shows that this new light source is a reliable and efficient alternative to an ordinary arc lamp excitation source for Fura-2 based ratio-metric fluorescence microscopy imaging.”
Research Paper: M. Chenchiliyan et al., "Dynamic
Ratiometric Imaging of Cytosolic Free Ca2+ in
Skeletal Muscle Cells Using 340/385-nm LightEmitting Diode Illuminators," in
IEEE Photonics
Journal, vol. 10, no. 6, pp. 1-10, Dec. 2018, Art no.
3700110; doi: 10.1109/JPHOT.2018.2882503
Read more:
Collimated LED Light Sources for Microscopy MicLED Light Source Series


These images show skeletal muscles stained with Fura-2 upon excitation with 385-nm LED light (top) and 340-nm LED light (bottom). Courtesy of Manoop Chenchiliyan, Bar-Ilan University.

